BLADDER CANCER STUDIES
METASTATIC BLADDER CANCER
## Cisplatin treatment superior
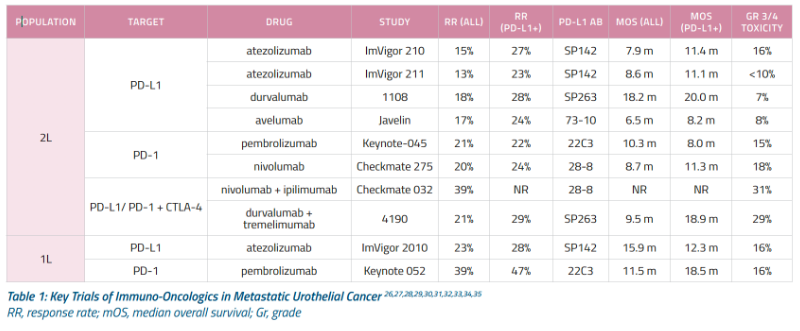
DeSantis et all - In cisplatin ineligible patients, 42% overall response (OR) w/ gemcitabine/carboplatin and 30% for methotrexate/carboplatin/vinblastine. However, if GFR <60ml/min and unfit, OR dropped to 26% and 30% respectively with increased toxicity. (16)
IMvigor 130, KEYNOTE - 361 - Based on the data from these studies the FDA issued a May 2018 safety alert that people with low PD-L1 expression have decreased survival with pembrolizumab or atezolizumab compared to cisplatin or carboplatin based therapy. In August they added the requirement for a diagnostic test be done in anyone on these drugs to test for PD-L1 expression.(11) These agents should only be used if cisplatin inelligible and low PD-L1 expression, as of September 2018 NCCN. (15)
KEYNOTe - 052 - Pembrolizumab as 1st line for cisplatin ineligible mBC showed 24% overall response with 5% complete response. (13)
IMvigor 210 - Atezolizumab as 1st line for cisplatin ineligible mBC showed 23% objective response rate with 9% complete response and median S of 15.9mo. (12)
AFTER FAILED PLATINUM THERAPY for MIBC
KEYNOTE 045 - After failing platinum based chemotherapy, pembrolizumab shown to be superior to investigator chosen chemotherapy (paclitaxel, docetaxil, venfluninine) in locally advanced and metastatic bladder cancer and to have a better side effect profile. Approved for 2nd line treatment of bladder cancer. Median survival 10.3mo vs. 7.3mo for chemotherapy. (8)
IMvigor-211 - After failing platinum based chemotherapy, atezolizumab shown to have no significant difference to investigator chosen chemotherapy (paclitaxel, docetaxil, venfluninine) in locally advanced and metastatic bladder cancer and to have a better side effect profile. Still approved for 2nd line treatment of bladder cancer. Median survival 11.1mo vs. 710.6mo for chemotherapy. (14)
Nivolumab, durvulamab, avelumab have favorable efficacy and safety from phase II trials but phase III trials are not yet reported and NCCN guidelines designated them as alternatives after failed platinum therapy, as of September 2018. (15)
NEOADJUVANT CHEMOTHERAPY for MIBC
NORDIC I and II - randomized: multiagent cisplatin NAC + RC vs. RC alone or RC + radiation
Showed neoadjuvant chemotherapy plus cystectomy extended OS vs. cystectomy alone
NEOADJUVANT CHEMO WITH SURVEILLANCE INSTEAD OF CYSTECTOMY
A retrospective review showed of 148 patients at a median of 55 (5-145) months, the 5yr disease-specific survival was 90%, overall was 85%, cystectomy-free 76%, and recurrence-free survival rate was 64% with 48% patients recurred in the bladder with 11% with muscle-invasive disease and 37% with non-invasive disease. Salvage radical cystectomy prevented cancer-specific death in 9 of 12 (75%) patients who accepted cystectomy after muscle-invasive relapse and 13 of 14 (93%) after non-invasive relapse. (10)
ADJUVANT CHEMOTHERAPY FOR MIBC - No real landmark studies
EORTC - improvement in progression free survival 3.11 vs. 0.99, however study closed early and was no impact on OS
OTHERS:
One study found patients with N+ disease had improved survival with cystectomy and chemotherapy in a retrospective study of 1,739 patients (cN1, 48%; cN2, 45%; cN3, 7%), of which 1,104 underwent cystectomy and 635 were treated with chemotherapy alone. Of the cystectomy patients, 363 received preoperative and 328 received adjuvant chemotherapy. The crude 5-year OS for chemotherapy alone, cystectomy alone, preoperative chemotherapy followed by cystectomy, and cystectomy followed by adjuvant chemotherapy was 14% (95% CI, 11% to 17%), 19% (95% CI, 15% to 24%), 31% (95% CI, 25% to 38%), and 26% (95% CI, 21% to 34%), respectively. Compared with cystectomy alone, preoperative chemotherapy was associated with a significant improvement in OS (hazard ratio, 0.80; 95% CI, 0.66 to 0.97). Adjuvant chemotherapy was also associated with a significant improvement in survival compared with cystectomy alone. The survival of patients treated with chemotherapy alone was worse than those treated with cystectomy alone. (9)
Multiple studies have found 5α reductase inhibitors were associated with better survival in bladder cancer. (17-20)
With absolute 3-year follow-up, 75% of patients with asymptomatic bacteruria survived tumor-free compared to 40% of 187 uninfected patients (P = .001). (21)
## Cisplatin treatment superior
DeSantis et all - In cisplatin ineligible patients, 42% overall response (OR) w/ gemcitabine/carboplatin and 30% for methotrexate/carboplatin/vinblastine. However, if GFR <60ml/min and unfit, OR dropped to 26% and 30% respectively with increased toxicity. (16)
IMvigor 130, KEYNOTE - 361 - Based on the data from these studies the FDA issued a May 2018 safety alert that people with low PD-L1 expression have decreased survival with pembrolizumab or atezolizumab compared to cisplatin or carboplatin based therapy. In August they added the requirement for a diagnostic test be done in anyone on these drugs to test for PD-L1 expression.(11) These agents should only be used if cisplatin inelligible and low PD-L1 expression, as of September 2018 NCCN. (15)
KEYNOTe - 052 - Pembrolizumab as 1st line for cisplatin ineligible mBC showed 24% overall response with 5% complete response. (13)
IMvigor 210 - Atezolizumab as 1st line for cisplatin ineligible mBC showed 23% objective response rate with 9% complete response and median S of 15.9mo. (12)
AFTER FAILED PLATINUM THERAPY for MIBC
KEYNOTE 045 - After failing platinum based chemotherapy, pembrolizumab shown to be superior to investigator chosen chemotherapy (paclitaxel, docetaxil, venfluninine) in locally advanced and metastatic bladder cancer and to have a better side effect profile. Approved for 2nd line treatment of bladder cancer. Median survival 10.3mo vs. 7.3mo for chemotherapy. (8)
IMvigor-211 - After failing platinum based chemotherapy, atezolizumab shown to have no significant difference to investigator chosen chemotherapy (paclitaxel, docetaxil, venfluninine) in locally advanced and metastatic bladder cancer and to have a better side effect profile. Still approved for 2nd line treatment of bladder cancer. Median survival 11.1mo vs. 710.6mo for chemotherapy. (14)
Nivolumab, durvulamab, avelumab have favorable efficacy and safety from phase II trials but phase III trials are not yet reported and NCCN guidelines designated them as alternatives after failed platinum therapy, as of September 2018. (15)
NEOADJUVANT CHEMOTHERAPY for MIBC
NORDIC I and II - randomized: multiagent cisplatin NAC + RC vs. RC alone or RC + radiation
- Multi-agent cisplatin NAC showed no benefit compared to cystectomy or cystectomy + radiation (- 1,2)
- However, when considering both trials together, NAC had 8% absolute risk reduction and 20% relative risk of death. (3)
- 145 deaths out of 306 in NAC group, 173 of 314 in control group at 5 years
- Initially showed 3 year nonsignificant survival benefit of 5.5% for NAC (4)
- F/u showed 10 year OS benefit of 6% (30vs 36%) for NAC CMV (5)
- 1% mortality from NAC, 2.7% from surgery
- Included T2 grade 3, T3-T4N0M0
- NAC + RC patients had 32.5% with pT0 post cystectomy; RC along had 12.3% pT0 (4)
- Downsides to this trial:
- CMV is no longer used and there is no trial comparing to MVAC
- RC vs. RT has not been compared in prospective randomized trials
Showed neoadjuvant chemotherapy plus cystectomy extended OS vs. cystectomy alone
- Median survival 77mo w/ NAC vs. 46mo w/o
- 5 year OS 57% w/ NAC vs 43% w/o
- 33% grade 4 granulocytopenia and 17% w/ grade 3 GI toxicities
- Included T2-T4aN0M0
- No deaths caused by NAC(6)
- NAC increased 5 year OS from 45% to 50%
- RC alone, RT alone and RC+RT were not superior to each other
- Single agent platinum NAC did not provide a survival benefit (7)
NEOADJUVANT CHEMO WITH SURVEILLANCE INSTEAD OF CYSTECTOMY
A retrospective review showed of 148 patients at a median of 55 (5-145) months, the 5yr disease-specific survival was 90%, overall was 85%, cystectomy-free 76%, and recurrence-free survival rate was 64% with 48% patients recurred in the bladder with 11% with muscle-invasive disease and 37% with non-invasive disease. Salvage radical cystectomy prevented cancer-specific death in 9 of 12 (75%) patients who accepted cystectomy after muscle-invasive relapse and 13 of 14 (93%) after non-invasive relapse. (10)
ADJUVANT CHEMOTHERAPY FOR MIBC - No real landmark studies
EORTC - improvement in progression free survival 3.11 vs. 0.99, however study closed early and was no impact on OS
OTHERS:
One study found patients with N+ disease had improved survival with cystectomy and chemotherapy in a retrospective study of 1,739 patients (cN1, 48%; cN2, 45%; cN3, 7%), of which 1,104 underwent cystectomy and 635 were treated with chemotherapy alone. Of the cystectomy patients, 363 received preoperative and 328 received adjuvant chemotherapy. The crude 5-year OS for chemotherapy alone, cystectomy alone, preoperative chemotherapy followed by cystectomy, and cystectomy followed by adjuvant chemotherapy was 14% (95% CI, 11% to 17%), 19% (95% CI, 15% to 24%), 31% (95% CI, 25% to 38%), and 26% (95% CI, 21% to 34%), respectively. Compared with cystectomy alone, preoperative chemotherapy was associated with a significant improvement in OS (hazard ratio, 0.80; 95% CI, 0.66 to 0.97). Adjuvant chemotherapy was also associated with a significant improvement in survival compared with cystectomy alone. The survival of patients treated with chemotherapy alone was worse than those treated with cystectomy alone. (9)
Multiple studies have found 5α reductase inhibitors were associated with better survival in bladder cancer. (17-20)
With absolute 3-year follow-up, 75% of patients with asymptomatic bacteruria survived tumor-free compared to 40% of 187 uninfected patients (P = .001). (21)
- Malmstrom, Per-Uno, et al. "Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: Nordic Cystectomy Trial 1." The Journal of urology 155.6 (1996): 1903-1906.
- Sherif, Amir, et al. "Neoadjuvant cisplatin-methotrexate chemotherapy for invasive bladder cancer-Nordic cystectomy trial 2." Scandinavian journal of urology and nephrology 36.6 (2002): 419-425.
- Sherif, Amir, et al. "Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies." European urology 45.3 (2004): 297-303.
- "International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial." Journal of Clinical Oncology 29, no. 16 (2011): 2171-2177.
- Sylvester, R., and C. Sternberg. "The role of adjuvant combination chemotherapy after cystectomy in locally advanced bladder cancer: what we do not know and why." Annals of Oncology 11.7 (2000): 851-856.
- Grossman, H. Barton, et al. "Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer." New England Journal of Medicine 349.9 (2003): 859-866.
- Vale, C., and Advanced Bladder Cancer (ABC) Meta-analysis Collaboration. "Neoadjuvant chemotherapy in invasive bladder cancer: a systematic review and meta-analysis." The Lancet 361.9373 (2003): 1927-1934.
- Bellmunt, Joaquim, et al. "Pembrolizumab as second-line therapy for advanced urothelial carcinoma." New England Journal of Medicine 376.11 (2017): 1015-1026.
- Galsky, Matthew D., et al. "Comparative effectiveness of treatment strategies for bladder cancer with clinical evidence of regional lymph node involvement." Journal of Clinical Oncology 34.22 (2016): 2627.
- Mazza, Patrick, et al. "Conservative Management Following Clinical Complete Response to Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: Contemporary Outcomes of a Multi-Institutional Cohort Study." The Journal of urology(2018).
- US FDA. FDA Alerts Health Care Professionals and Oncology Clinical Investigators About and Efficacy Issue Identified in Clinical Trials or Some Patients Taking Keytruda (pemnrolizumab) ot Tecentriq (atezolizumab) as Monotherapy to Treat Urothelial Cancer With Low Expression of PD-La. Updated August 16, 2018. Acessed September 30, 2018.
- Balar, Arjun V., et al. "Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial." The Lancet 389.10064 (2017): 67-76.
- Balar, Arjun V., et al. "First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study." The Lancet Oncology 18.11 (2017): 1483-1492.
- Rosenberg, Jonathan E., et al. "Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial." The Lancet 387.10031 (2016): 1909-1920.
- Flaig, Thomas W., et al. "NCCN Guidelines Insights: Bladder Cancer, Version 5.2018." Journal of the National Comprehensive Cancer Network 16.9 (2018): 1041-1053.
- De Santis, Maria, et al. "Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986." Journal of clinical oncology 30.2 (2012): 191.
- Mäkelä, Ville J., et al. “Bladder Cancer Survival of Men Receiving 5α-Reductase Inhibitors.” The Journal of Urology, vol. 200, no. 4, 2018, pp. 743–748.
- Morales, Edwin E., et al. “Finasteride Reduces Risk of Bladder Cancer in a Large Prospective Screening Study.” European Urology, vol. 69, no. 3, 2016, pp. 407–410.
- Chen, C. C., Huang, C. P., Tsai, Y. T., Hseih, T. F., & Shyr, C. R. (2017). The genomic alterations of 5α-reductases and their inhibitor finasteride's effect in bladder cancer. Anticancer research, 37(12), 6893-6898.
- Dallob, AiMfiE L., et al. “The Effect of Finasteride, a 5 Alpha-Reductase Inhibitor, on Scalp Skin Testosterone and Dihydrotestosterone Concentrations in Patients with Male Pattern Baldness.” The Journal of Clinical Endocrinology and Metabolism, vol. 79, no. 3, 1994, pp. 703–706.
- Herr, Harry, and Machele Donat. "Reduced Recurrence of Low-grade Papillary Bladder Tumors Associated With Asymptomatic Bacteriuria." Urology 124 (2019): 179-182.